Discovery and development of “cocktail”-type catalysis
15.05.2025
Discovery and development of “cocktail”-type catalysis
From the proceedings of the conference “New Horizons of Catalysis and Organic Chemistry”, ZIOC RAS, 2025.
Catalysis plays a central role in modern chemistry, allowing reactions to occur faster, cleaner, and more efficiently. From making medicines and fertilizers to producing plastics and fuels, catalysts are behind countless processes that shape our daily lives. Traditionally, chemists have understood catalysis in two main forms: homogeneous, where the catalyst exists in the same phase as the reactants and heterogeneous, where the catalyst represents a separate phase, such as a supported solid that acts on reactants in liquid or gaseous form. For decades, these two types of catalysis were treated as separate worlds.
But in recent years, scientists have uncovered a much more dynamic and fascinating picture. Rather than being fixed in a single form, catalysts can change during the reaction - they can evolve and adapt, transforming into entirely new species. This idea has led to a new concept called “cocktail”-type catalysis (Figures 1 and 2). Just as a cocktail combines different ingredients into a flavorful mix, catalytic systems can contain a blend of different active forms: molecular complexes, metal clusters, and nanoparticles. These forms do not just coexist - they can actively transform into one another depending on the temperature, solvent, or other reaction conditions.
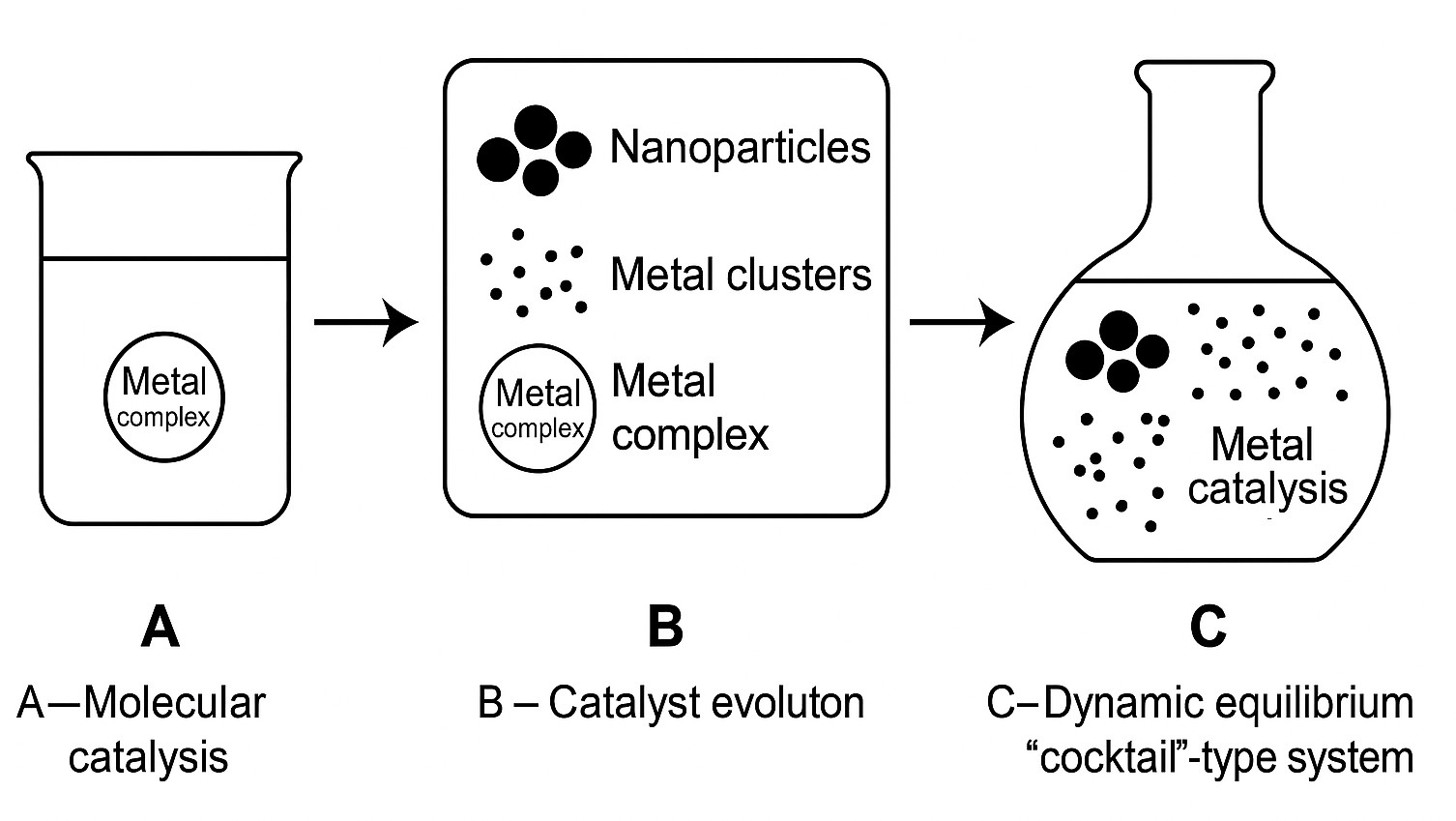
 Figure 1. Bottom-up pathway to "cocktail"-type catalysis concept through three labeled stages. A – Molecular catalysis: a precursor contains a single metal complex, representing a traditional homogeneous catalyst; B – Catalyst evolution: a transformation stage where the metal complex gives rise to various species, including metal nanoparticles, metal clusters, and complexes; C – Dynamic equilibrium "cocktail"-type system: a real system contains a mixture of all species (complexes, nanoparticles, clusters), illustrating a dynamic, equilibrated reaction environment with multiple coexisting catalytically active forms.
Figure 1. Bottom-up pathway to "cocktail"-type catalysis concept through three labeled stages. A – Molecular catalysis: a precursor contains a single metal complex, representing a traditional homogeneous catalyst; B – Catalyst evolution: a transformation stage where the metal complex gives rise to various species, including metal nanoparticles, metal clusters, and complexes; C – Dynamic equilibrium "cocktail"-type system: a real system contains a mixture of all species (complexes, nanoparticles, clusters), illustrating a dynamic, equilibrated reaction environment with multiple coexisting catalytically active forms.
Imagine starting a reaction with what seems to be a well-defined palladium complex (Figure 1). As the reaction proceeds, some molecules might break apart, forming tiny clusters of atoms, or growing into nanoparticles. Some of these forms may catalyze certain steps of the reaction of interest, while others take care of the rest. For instance, in some cases, nanoparticles release metal atoms into the solution, which become active on their own. Later, these atoms may regroup into particles again. It is a chemical system in motion, which can also arise with the supported nanoparticles as a catalyst precursor (Figure 2).
This concept helps explain why some reactions are more efficient than expected, or why even tiny traces of a metal can result in full conversion of substrates. “Cocktail”-type systems are often more robust and adaptive than single-component catalysts. If one form is deactivated, another can take over. Some species act as reservoirs, releasing or recapturing metal atoms as needed. This adaptability makes such systems particularly useful for green chemistry, where minimizing waste and using fewer precious metals are top priorities.
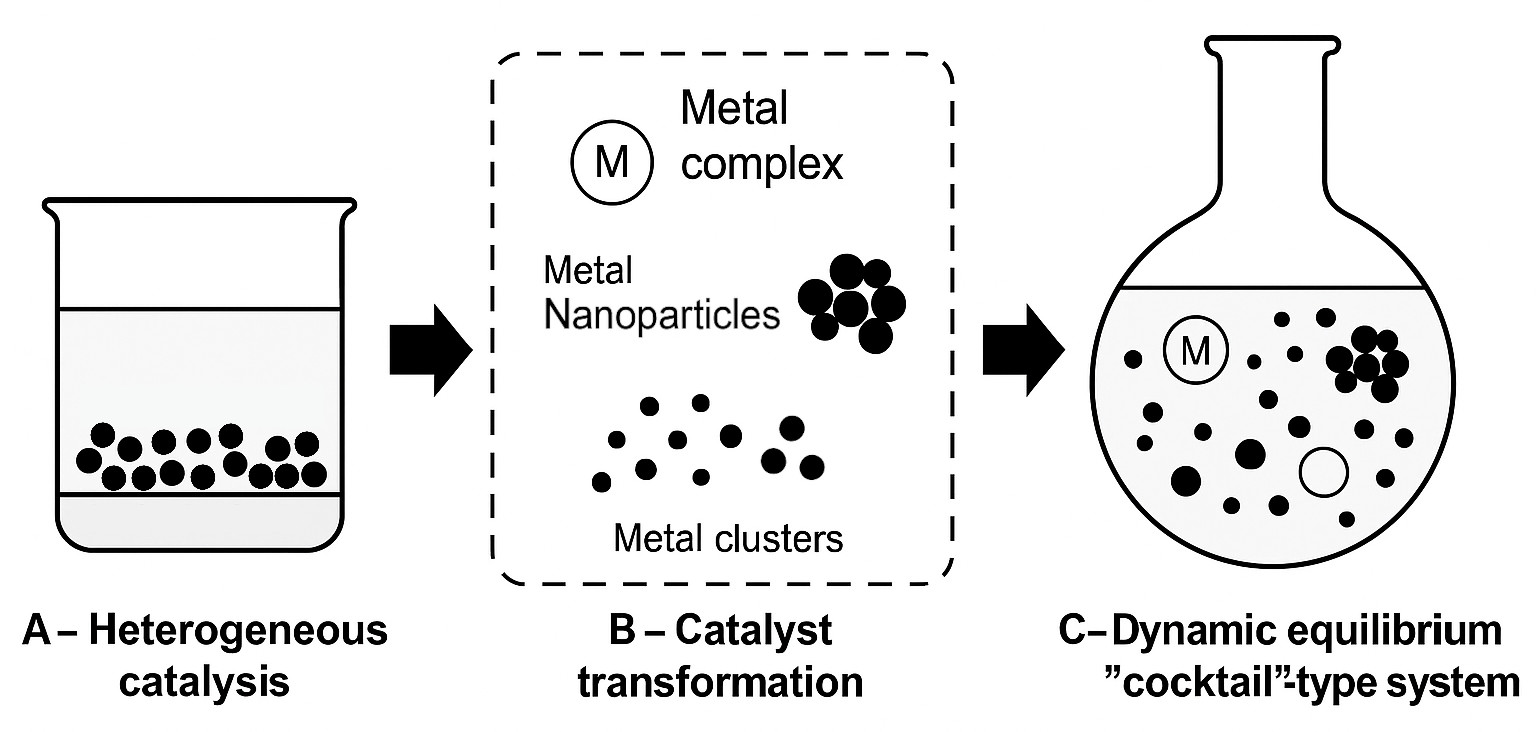
Figure 2. Top-down pathway to “cocktail”-type catalysis. A – heterogeneous catalysis begins with a supported metal catalyst; B – catalyst transformation involves leaching, nanoparticle formation, cluster growth, and emergence of soluble metal complexes; C – a dynamic equilibrium “cocktail”-type system is established, where various metal-containing species coexist and contribute to catalysis.
The “cocktail-type catalysis” was first described in 2012 by Valentine Ananikov and his colleagues (http://AnanikovLab.ru). They discovered that a widely used palladium catalyst, long thought to be purely molecular, actually contained palladium nanoparticles that played a key role in the reaction. Since then, many other systems based on various metals – platinum, rhodium, nickel, copper and others - have been shown to follow similar behavior. The idea has grown into a broader understanding that real catalytic systems are rarely static; they are often a dynamic mix of different forms, cooperating in a kind of chemical equilibrium.
For students and researchers, this concept is both exciting and illuminating. It shows that real-world chemistry does not always follow obvious textbook models. Instead of one perfectly stable catalyst, we may be dealing with a team of catalysts that adapt and evolve as the reaction unfolds. It bridges the gap between homogeneous and heterogeneous catalysis and brings a fresh perspective on how molecular structure, particle size, and surface interactions come together to drive chemical transformations.
“Cocktail”-type catalysis gives rise to a new way of thinking. Rather than fighting complexity, it embraces it. This approach is helping chemists design better, more flexible catalytic systems - ones that work under challenging conditions, require less metal, and offer greater control over reactivity and selectivity. It is a dynamic, cooperative view of catalysis that opens the door to next-generation technologies in sustainable chemistry and industrial applications.
References
Discovery of “cocktail”-type effect:
- Zalesskiy S.S., Ananikov V.P., "Pd2(dba)3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis", Organometallics, 2012, 31, 2302-2309. http://dx.doi.org/10.1021/om201217r
Key reviews:
- Ananikov V.P., Beletskaya I.P., "Toward the Ideal Catalyst: From Atomic Centers to a "Cocktail" of Catalysts", Organometallics, 2012, 31, 1595-1604. http://dx.doi.org//10.1021/om201120n
- Kashin A.S., Ananikov V.P., "Catalytic C-C and C-Heteroatom Bond Formation Reactions: In Situ Generated or Preformed Catalysts? Complicated Mechanistic Picture Behind Well-Known Experimental Procedures", J. Org. Chem., 2013, 78, 11117-11125. http://dx.doi.org/10.1021/jo402038p
- Eremin D.B., Ananikov V. P., "Understanding Active Species in Catalytic Transformations: from Molecular Catalysis to Nanoparticles, Leaching, “Cocktails” of Catalysts and Dynamic Systems", Coord. Chem. Rev., 2017, 346, 2-19. http://dx.doi.org/10.1016/j.ccr.2016.12.021
- Prima D.O., Kulikovskaya N.S., Galushko A.S., Mironenko R.M., Ananikov V.P. "Transition metal “cocktail”-type catalysis", Curr. Opinion in Green and Sustainable Chemistry 2021, 100502. https://doi.org/10.1016/j.cogsc.2021.100502
- Chernyshev V.N., Denisova E.A., Eremin D.B., Ananikov V. P., "The key role of R-NHC couplings (R = C, H, heteroatom) and M-NHC bond cleavage in the evolution of M/NHC complexes and formation of catalytically active species", Chem. Sci., 2020, 11, 6957-6977. https://doi.org/10.1039/D0SC02629H
Adaptive catalysis:
- Ananikov V. P., Orlov N. V., Zalesskiy S. S., Beletskaya I. P., Khrustalev V. N., Morokuma K., Musaev D. G., "Catalytic Adaptive Recognition of Thiol (SH) and Selenol (SeH) Groups Towards Synthesis of Functionalized Vinyl Monomers", J. Am. Chem. Soc., 2012, 134, 6637-6649. http://dx.doi.org/10.1021/ja210596w
- Ghosh I., Shlapakov N.S., Karl T.A., Düker J., Nikitin M., Burykina J.V., Ananikov V.P., König B. "General cross-coupling reactions with adaptive dynamic homogeneous catalysis", Nature, 2023, 619, pages 87–93. https://doi.org/10.1038/s41586-023-06087-4
- Kashin A.S., Arkhipova D.M., Sahharova L.T., Burykina J.V., Ananikov V.P., "Revealing Catalyst Self-Adjustment in C–S Cross-Coupling through Multiscale Liquid-Phase Electron Microscopy", ACS Catal., 2024, 14, 8, 5804–5816. https://doi.org/10.1021/acscatal.3c06258
Development and examples of “cocktail”-type systems:
- Prima D.O., Kulikovskaya N.S., Novikov R.A., Kostyukovich A.Yu., Burykina J.V., Chernyshev V.N., Ananikov V. P., "Revealing the mechanism of combining best properties of homogeneous and heterogeneous catalysis in hybrid Pd/NHC systems", Angew. Chem. Int. Ed., 2024, 63 (27), e202317468. https://doi.org/10.1002/anie.202317468
- Galushko A.S., Boiko D.A., Pentsak E.O., Eremin D.B., Ananikov V. P. "Time-Resolved Formation and Operation Maps of Pd Catalysts Suggest a Key Role of Single Atom Centers in Cross-Coupling", J. Am. Chem. Soc., 2023, 145, 16, 9092-9103. https://doi.org/10.1021/jacs.3c00645
- Eremin D.B., Galushko A.S., Boiko D.A., Pentsak E.O., Chistyakov I.V., Ananikov V. P., "Toward Totally Defined Nanocatalysis: Deep Learning Reveals the Extraordinary Activity of Single Pd/C Particles", J. Am. Chem. Soc., 2022, 144, 13, 6071–6079. https://doi.org/10.1021/jacs.2c01283
- Astakhov A.V., Khazipov O.V., Chernenko A.Yu., Pasyukov D.V., Kashin A.S., Gordeev E.G., Khrustalev V.N., Chernyshev V.M., Ananikov V.P., "A New Mode of Operation of Pd-NHC Systems Studied in a Catalytic Mizoroki–Heck Reaction", Organometallics, 2017, 36, 1981–1992. http://dx.doi.org/10.1021/acs.organomet.7b00184
- Panova Yu.S., Kashin A.S., Vorobev M.G., Degtyareva E.S., Ananikov V.P., "Nature of the Copper-Oxide-Mediated C−S Cross-Coupling Reaction: Leaching of Catalytically Active Species from the Metal Oxide Surface", ACS Catal., 2016, 6, 3637 – 3643. http://dx.doi.org/10.1021/acscatal.6b00337
- Ondar E.O., Kostyukovich A.Yu., Burykina J.V., Galushko A.S., Ananikov V. P., "Examination of Pt2dba3 as a “cocktail”-type catalytic system for alkene and alkyne hydrosilylation reactions", Catal. Sci. Technol., 2023, 13, 6022-6040. https://doi.org/10.1039/D3CY00865G




